Summary
- The QM atom
- L-S coupling and J
- Lasers
Chapter 12
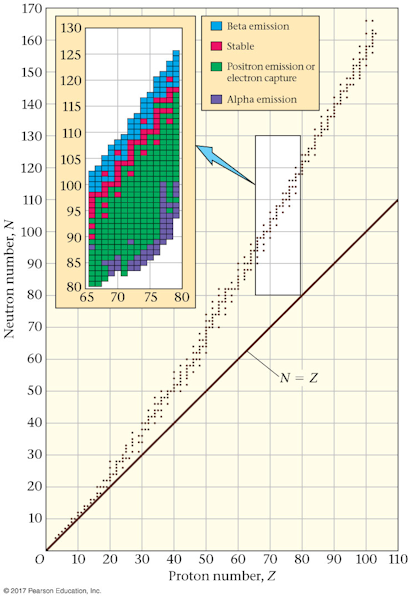
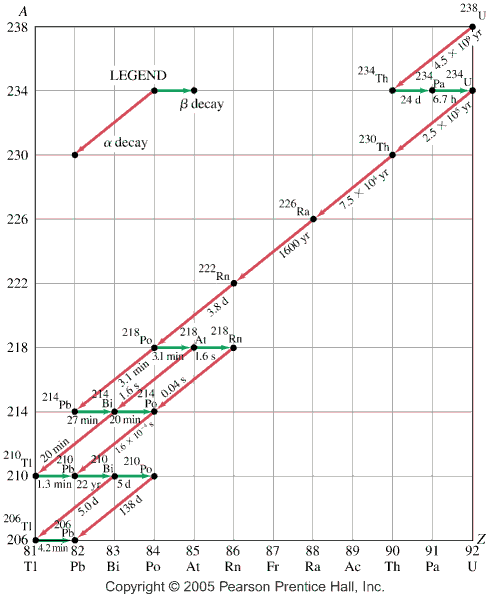
- The nucleus
- protons
- neutrons
- isotopes
- table of isotopes applet
Example #1
- Example problem 12.03
- nuclear radius
- nuclear density
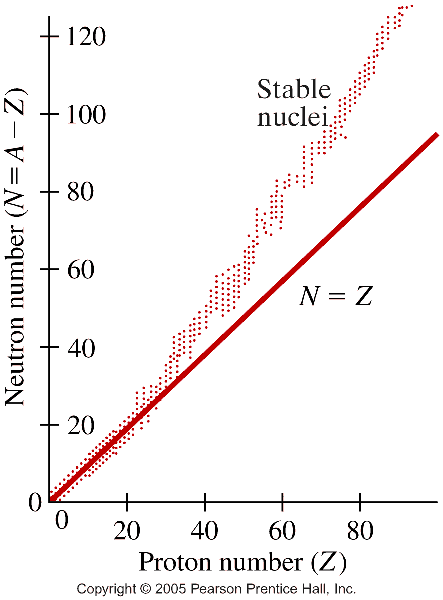
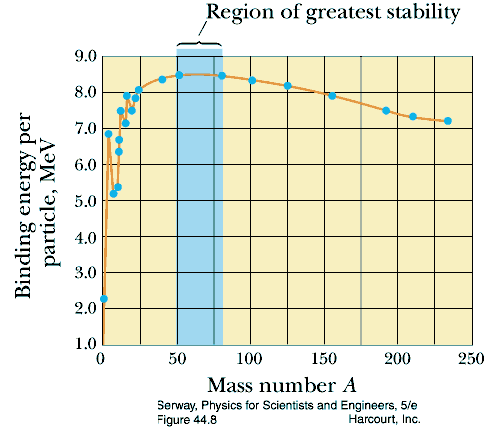
- Binding energy of the nucleus
- origin of the energy
- calculating B
- Example problem 12.15
- Example problem 12.18
- maximum energy
- stars & nuclear fusion
Example #2
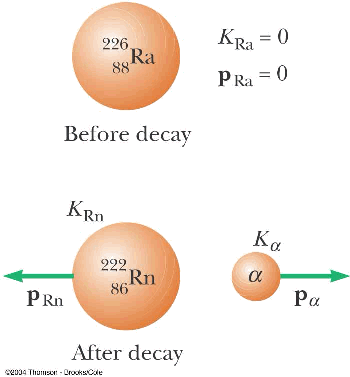
- Radioactive decay
- alpha decay
- beta minus decay
- gamma decay
Example #3
- Δm and the energy released
- Practice:
Try these additional examples
Example #4
Example #5
gc6 30.q2
What isotope is represented by the X in
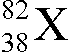 ?
?
A. xenon-82
B. lead-38
C. strontium-38
D. strontium-82
Answer
gc6 30.12
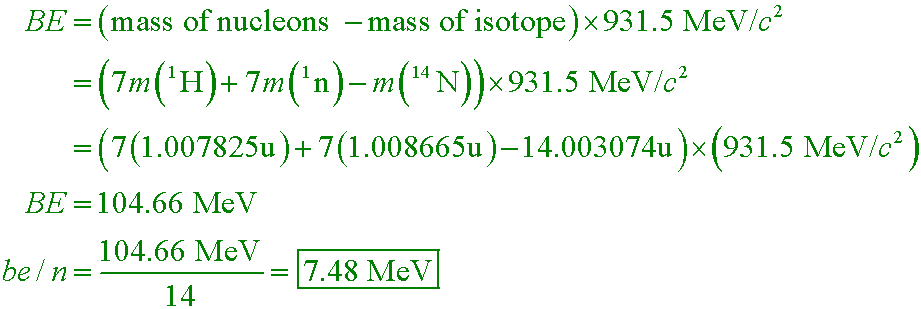
Use Appendix 8 to calculate the binding energy per nucleon for nitrogen-14.
A. 7.48 MeV
B. 0.112 MeV
C. 15.0 MeV
D. 105 MeV
Answer
gc6 30.23mod

Answer
Walker5e EYU 32.1
Consider a stable nucleus with a proton number of roughly 70. Rank the numbers N, Z, and A for this nucleus in order of increasing value. Indicate ties where appropriate.
1. Z < N = A
2. A = Z < N
3. Z < N < A
4. Z < A < N
Answer
Walker5e EYU 32.2 mod
A given nucleus can undergo alpha decay, beta decay, or gamma decay. Rank the masses A of the daughter isotopes for each case. Indicate ties where appropriate.
1. Aalpha < Abeta < Agamma
2. Agamma < Abeta < Aalpha
3. Agamma < Aalpha = Abeta
4. Aalpha = Abeta = Agamma
Answer
D. strontium-82
The number at the lower left is the atomic number Z and identifies
the isotope as strontium. The number at the upper left is the atomic number A and it tells you the
number of nucleons is 82.
A. 7.48 MeV

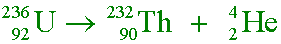
3. Z < N < A
For heavy nuclei the neutrons N outnumber the protons Z, and the mass number A is the sum of N and Z, so it is the largest of them all.
1. Aalpha < Abeta < Agamma
The alpha particle is the heaviest of the three, so alpha emission leaves behind the lightest daughter nucleus. The beta particle is very light, but the gamma photon is massless, so gamma emission leaves behind the heaviest daughter isotope of the three.





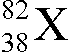 ?
?

