kw5
Which of the following is NOT a feature of the Bohr model of the atom?
A. Electrons in planetary-like orbits.
B. Positively charged material surrounding the electrons.
C. Quantized energy levels.
D. Accelerating electrons that do not radiate.
Answer
kw5
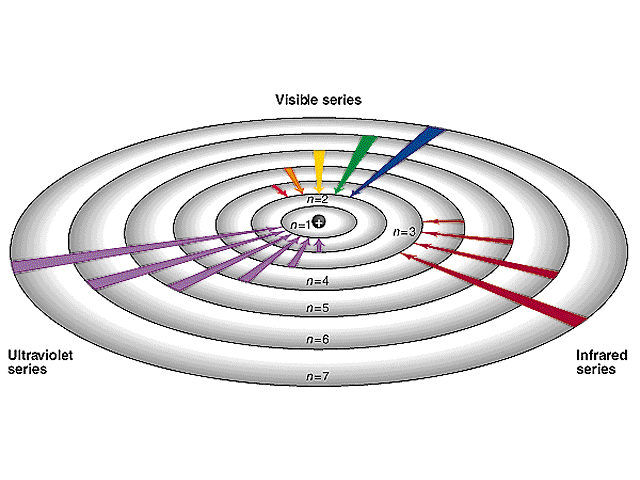
If the electrons in hydrogen atoms are excited to the fourth Bohr orbit, how many different frequencies of light may be emitted?
A. 1
B. 3
C. 4
D. 6
Answer
Walker5e EYU 31.3a
If the principal quantum number n is doubled in the Bohr model, the speed of an electron in an orbit _____.
A. is cut to a fourth
B. is cut in half
C. stays the same
D. doubles as well
E. is quadrupled
Answer
Walker5 31.29a
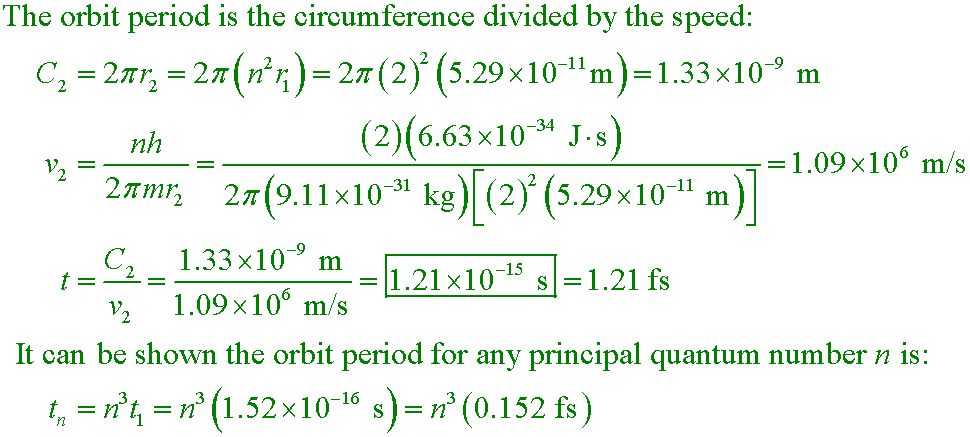
Calculate the time required for an electron in the n = 2 state of hydrogen to complete one orbit about the nucleus.
A. 1.21×10−15 s
B. 5.29×10−11 s
C. 9.11×10−9 s
D. 6.63×10−7 s
Answer
B. Positively charged material surrounding the electrons.
The Bohr model retains Rutherford's compact nucleus as the location of all the positive charge in an atom. The Thomson "plum pudding" model is the one that has a positively charged "pudding" surrounding the negatively charged electrons.
D. 6
If the electron jumps from the n = 4 to the n = 3 level, it can then also jump from
3 → 2, 3 → 1, or
2 → 1. Each of those four transitions would release a photon of a unique
energy or color. Two other unique colors would result from 4 → 2 and
4 → 1 jumps, for a total of 6 unique colors.
B. is cut in half
As seen in Equation 31-6, the speed of an electron in a Bohr orbit is inversely proportional to the principal quantum number n.

A. 1.21×10−15 s
Second part of the question:
(b) The typical "lifetime" of an electron in the n = 2 state is roughly
10−8 s; after this time the electron is likely to have dropped back to the
n = 1 state. Estimate the number of orbits an electron completes
in the n = 2 state before dropping to the ground state.