Summary
- Millikan oil drop experiment
- Blackbody radiation
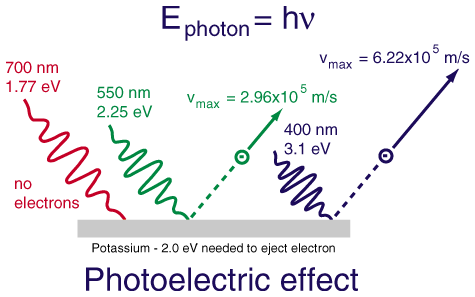
- The photoelectric effect applet
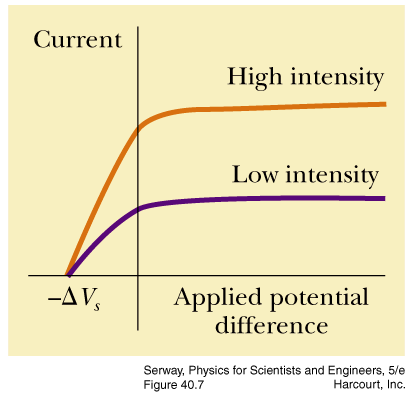
kw5
Light striking a clean metal produces photoelectrons. If the light is made twice as bright, what happens to the average kinetic energy of the photoelectrons?
A. The energy doubles.
B. The energy stays the same.
C. The energy is cut in half.
D. It's impossible to say.
Answer
kw5
Blue light shines on a metal, producing photoelectrons. When a red light is used the maximum KE of the photoelectrons
A. increases.
B. decreases.
C. stays the same.
D. Depends on the light intensity.
Answer
sj6 40.14
For a certain surface the emitted photoelectrons have vmax=460 km/s when l=625 nm.
What minimum wavelength produces photoelectrons?
A. 756 nm
B. 897 nm
C. 962 nm
D. 1064 nm
Answer
B. The energy stays the same.
The average kinetic energy of the emitted photoelectrons depends only upon the frequency of the light. Making the light brighter
produces more photoelectrons, but they have the same energy distribution as before.
B. decreases.
Red light is composed of photons that have a lower frequency and thus less energy than blue photons. The emitted photoelectrons
will therefore have less energy when red light is used.
B. 897 nm



