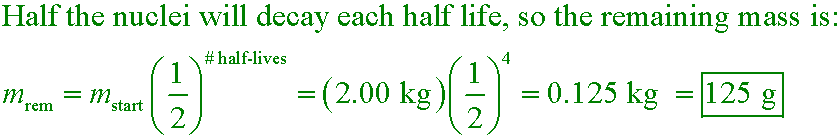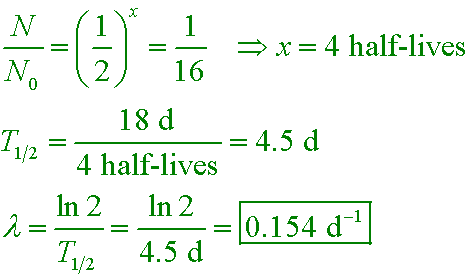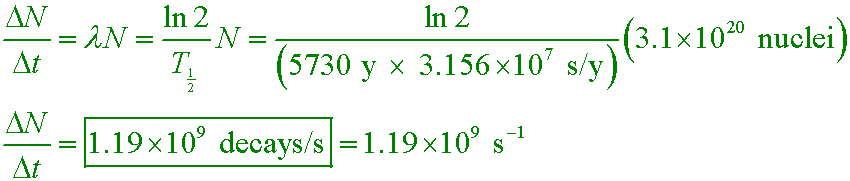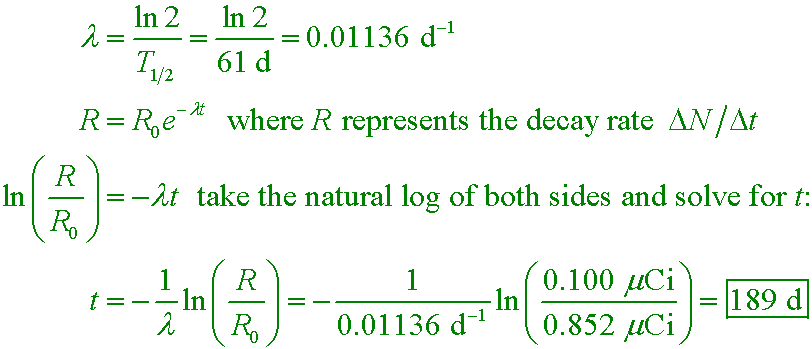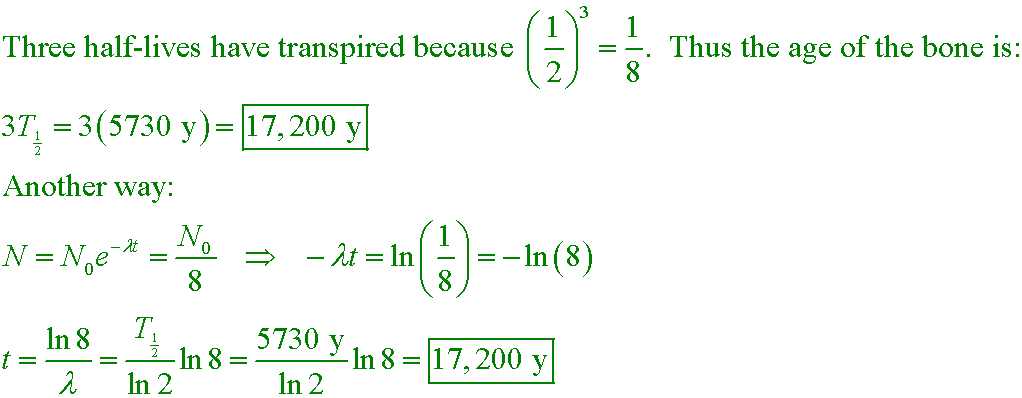Summary
- Lasers
- Structure of the nucleus
- Radioactive decay

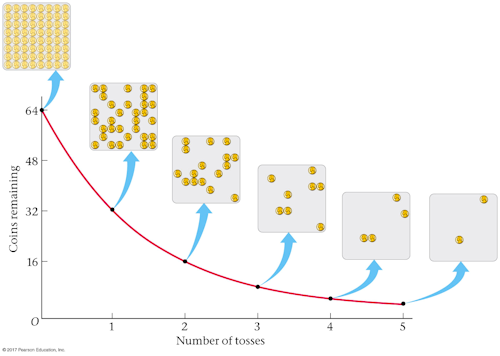
- Exponential decay
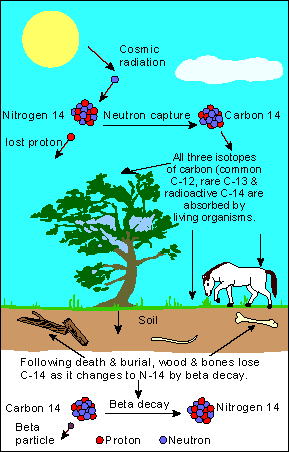
- Radioactive dating
- Lecture learning outcomes
A student who masters the topics in this lecture will be able to:
- describe the connection between the exponential decay of a radioactive isotope and its half-life.
- use algebra to find the number of remaining radioactive nuclei N, the initial number of radioactive nuclei N0, the decay constant λ, or time elapsed t for radioactive decay when any three of these quantities are given
- calculate the half-life T½ or the decay constant λ when either one of these quantities is known.
Practice:
Try these additional examples
Example #6
Example #7
Prepare:
Optional: Read textbook sections 32-4 through 32-7 before the next lecture
kw5
If you start with 2.00 kg of a radioactive substance, how much will you have after 4 half-lives?
A. 125 g
B. 500 g
C. 2.00 kg
D. 8.00 kg
Answer
Walker5e 32.33
The number of radioactive nuclei in a sample decreases over a period of 18 d to one-sixteenth the original
number. What is the decay constant?
A. 0.0556 d−1
B. 0.0625 d−1
C. 0.154 d−1
D. 0.693 d−1
Answer
gc6 30.38
What is the activity of a sample of carbon-14 that contains 3.1×1020 nuclei? The half-life is 5730 y.
A. 5.6×1031 s−1
B. 3.1×1020 s−1
C. 3.8×1016 s−1
D. 1.2×109 s−1
Answer
Walker5e 32.37
If a spiny lobster is given an activity of 0.852 µCi with technetium-95 (half-life 61 d), how much time will elapse before its activity falls below a 0.100 µCi detection limit?
A. 3.1 y
B. 189 d
C. 8.52 d
D. 20.4 h
Answer
kw5
If the ratio of 14C to 12C in a piece of bone is only 1/8 of the atmospheric ratio,
how old is the bone? The half-life of 14C is 5730 years.
A. 716 y
B. 22,920 y
C. 17,200 y
D. 45,800 y
Answer
Walker5e EYU 32.3 mod
A sample of radioactive isotope A and a sample of radioactive isotope B decay as shown in the graph below. Which sample exhibits the greater decay constant λ?
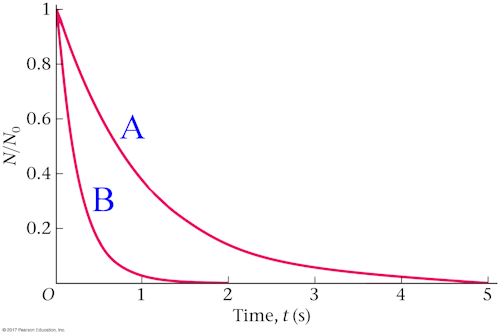 A. sample A
A. sample A
B. sample B
C. Their decay constants are the same.
Answer
Walker5e 32.29
Suppose we were to discover that the ratio of carbon-14 to carbon-12 in the atmosphere was significantly smaller 10,000 years ago than it is today. Would the true age of an object be greater than, less than, or equal to the age determined by using the standard carbon-14 dating method?
A. greater than
B. less than
C. equal to
Answer
A. 125 g
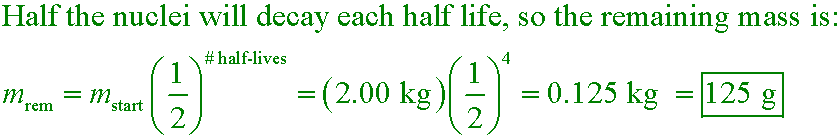
C. 0.154 d−1
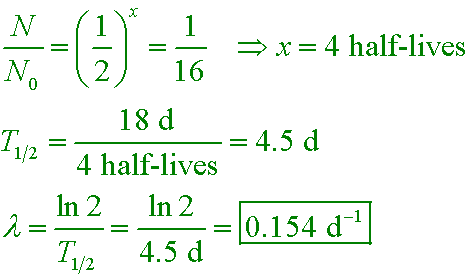
D. 1.2×109 s−1
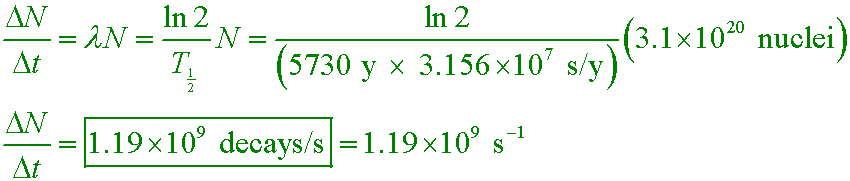
B. 189 d
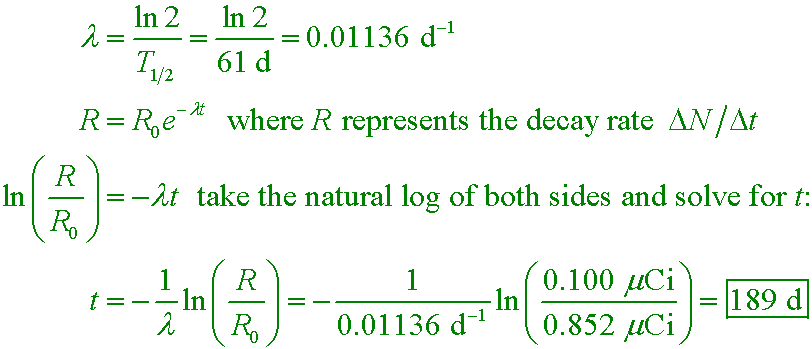
C. 17,200 y
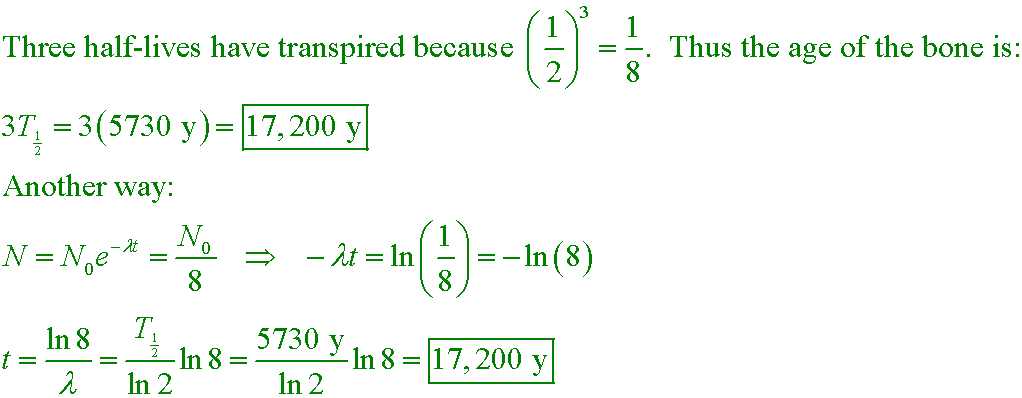

B. sample B
Sample B has the shorter half-life, hence it must have the larger decay constant because λ = (ln 2)/T½. Alternatively, the graph indicates a larger fraction of sample B nuclei decay every second, thus λB > λA because the decay constant represents the fraction of nuclei that decay each time interval.
B. less than
An organism that lived during the carbon-14-deficient era would have less carbon-14 incorporated into its tissues than an organism that lives today. This deficiency would make the ancient organism appear older than it really is, as if a longer time had passed and more of its carbon-14 nuclei had decayed.



 A. sample A
A. sample A