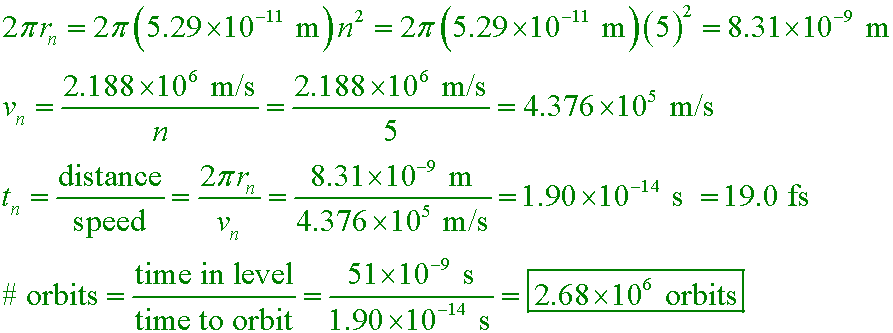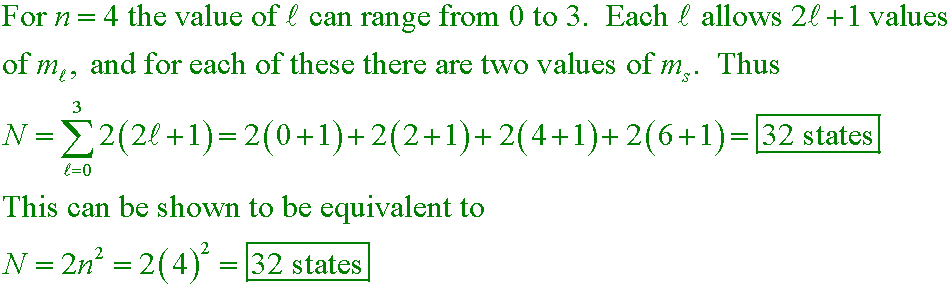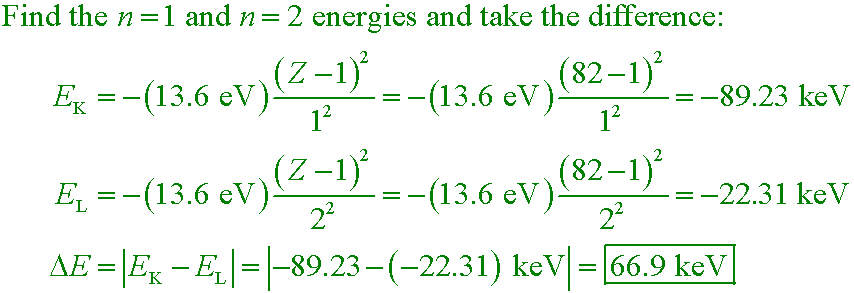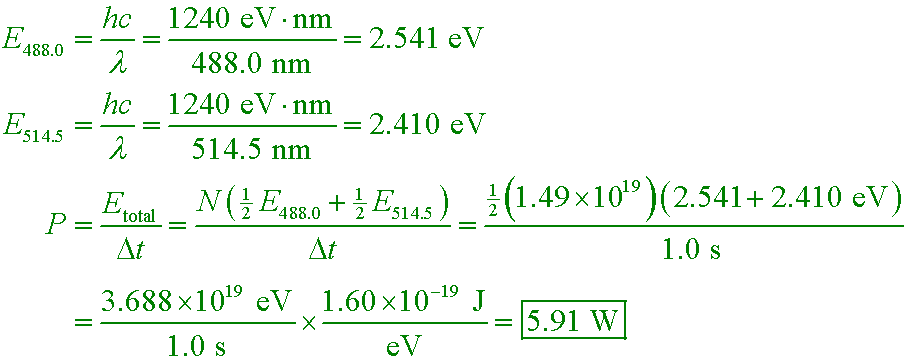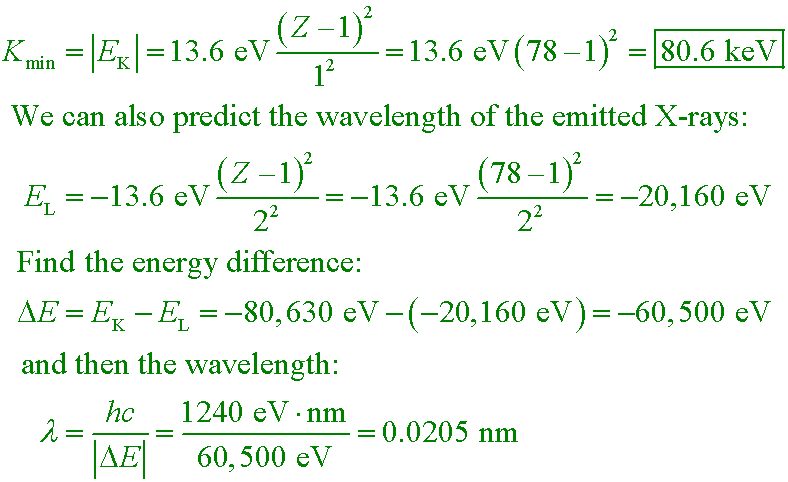Example #2
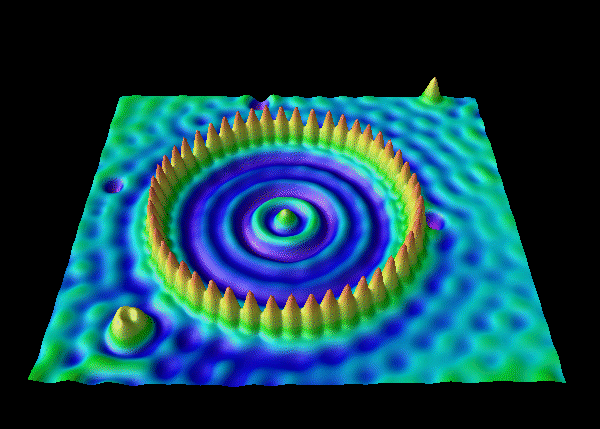
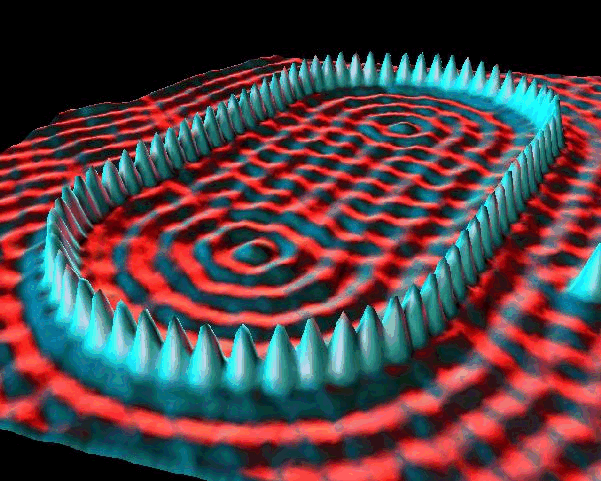
IBM researchers used a scanning tunneling microscope to arrange Xe atoms on a chilled Ni surface. (Discussion and video) The trapped electrons demonstrate their wave nature via Freidel oscillations. More info from UW Madison Materials Research.


- Schrödinger equation
- energy levels
- four quantum numbers
- The Pauli exclusion principle
- electron wave functions applet
- H orbitals
Example #3
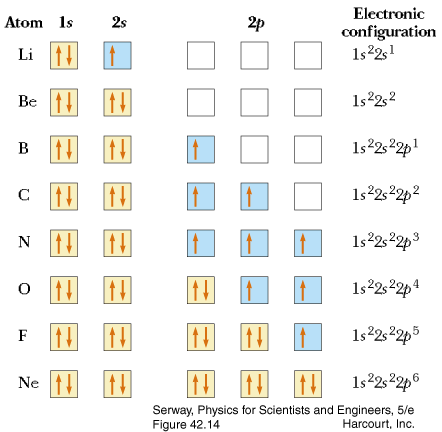
Example #4

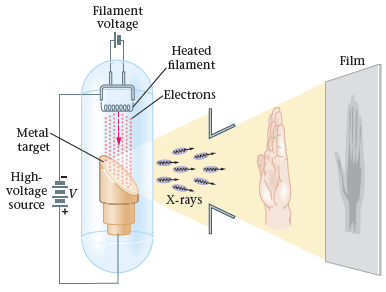
- X-ray tubes
- electron shielding
- approximate energy levels
Example #5
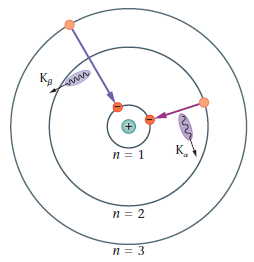
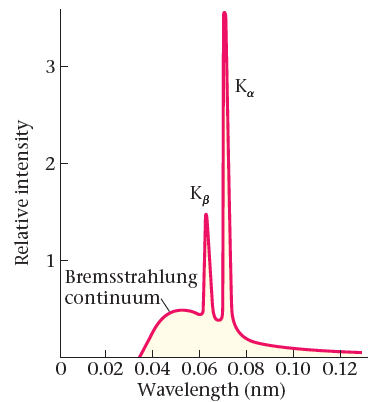
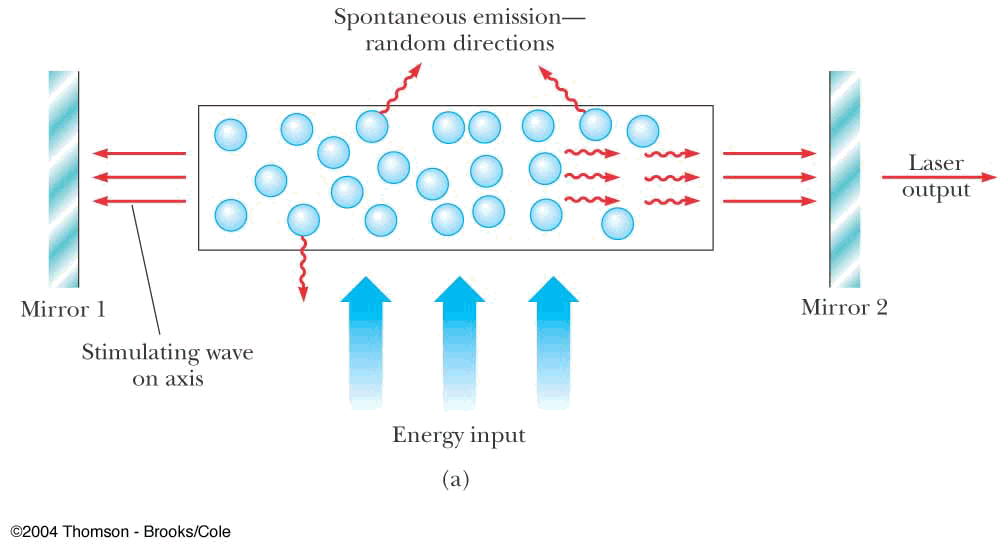
- spontaneous emission
- stimulated emission applet
- laser operation
Amplification by
Stimulated
Emission of
Radiation
Example #6
A student who masters the topics in this lecture will be able to:
- describe how de Broglie waves explained why electrons in allowed orbits do not radiate
- describe the basic features of the quantum mechanical model of the atom
- explain how an X-ray tube works
- calculate the wavelength of Kα X-rays for an element of atomic number Z
- describe the operating principles of a laser, and calculate the energy delivered by laser beam during a time interval Δt when given the beam intensity I and cross-sectional area A
 A. increase
A. increase