Summary
- Optical resolution
- Diffraction gratings
- Holography
Physical Chemistry (Ball) Ch. 19
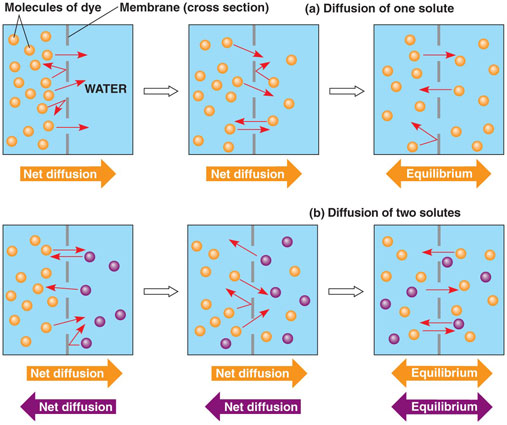

- gases
- solutes
- Fick's first law
- entropy
Example #1
Example #2
- Einstein-Smoluchowski
- one-dimensional diffusion
- three-dimensional diffusion
Example #3
Example #4
- Lecture learning outcomes
A student who masters the topics in this lecture will be able to:
- describe how a concentration gradient can be used to predict the rate at which molecules diffuse or the time required for an average molecule to diffuse a certain distance.
- use algebra to find the number ΔN of molecules that diffuse, the time interval Δt, the diffusion coefficient D, or the concentration gradient Δc/Δx for molecular diffusion when any three of these quantities are given
- use algebra to find the diffusion length Δx, the diffusion time t, or the diffusion coefficient D for either one-dimensional or three-dimensional molecular diffusion when any two of these quantities are given
Practice:
Try these additional examples
Example #5
Example #6
Prepare:
Read the textbook section on diffusion (on Canvas) before the next lecture
phys today 2013.July.p41
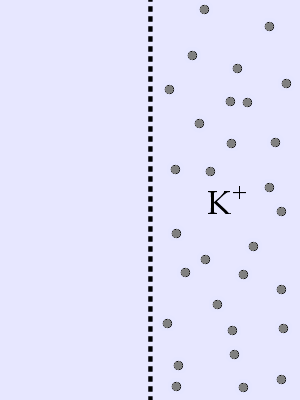
You have a membrane with pure water on the left side and K+ ions on the right
side. The membrane is permeable to K+. The K+ will _____ and will stop moving _____.
 A. move left ... after an infinite time
A. move left ... after an infinite time
B. move right ... when Δc = 0
C. move left ... when Δc = 0
D. not move ... (didn't move)
Answer
phys today 2013.July.p41
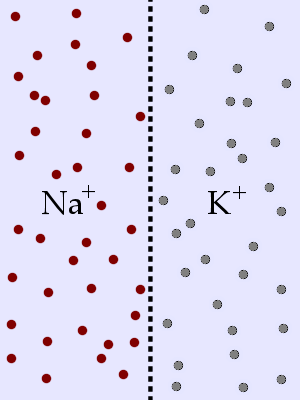
You have a membrane with Na+ ions on the left side and K+ ions on the right side (equal numbers of
ions on both sides). The membrane is permeable to K+ only. The K+ will _____ and will stop moving _____.
 A. move left ... after an infinite time
A. move left ... after an infinite time
B. move left ... when ΔcK+ = ΔcNa+
C. move left ... when ΔcK+ = 0
D. not move ... (didn't move)
Answer
Ball Example 19.9
Suppose some ammonia bleach is spilled on the floor 2.00 m away from where you are sitting. Assuming
that the molecules are transported solely by (three-dimensional) diffusion, how much time will elapse
before the average NH3 molecule will reach your nose? The diffusion coefficient for NH3
in air is 0.219 cm2/s.
A. 0.088 s
B. 30.4 s
C. 7.61 min
D. 8.46 h
Answer
UMD example
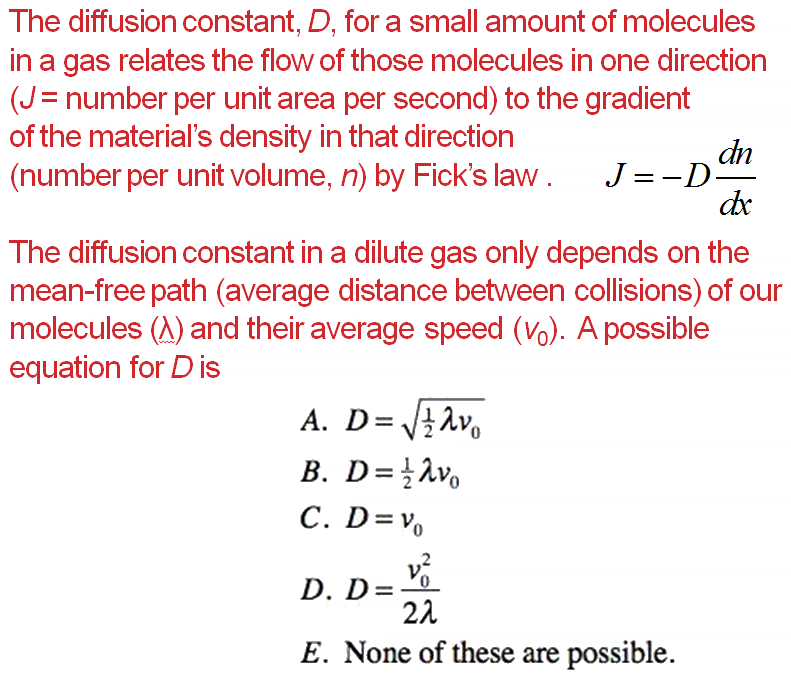
Answer
klm
An aquarium bubbler can generate concentration c0 of oxygen in its immediate surroundings. Assuming the bubbler is at one end of the tank and there is no oxygen at the other end of the tank, the oxygen diffusion rate it produces in a 30-cm-long tank is _____ the diffusion rate it produces in a 60-cm-long tank.
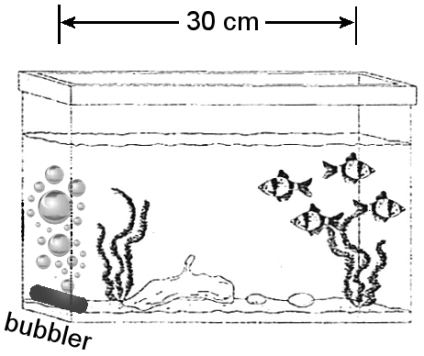 A. one-quarter
A. one-quarter
B. half
C. the same as
D. twice
E. four times
Answer
Ball 19.68
The diffusion coefficient of helium in neon is 31.2 cm²/s at 298 K. What is the average (root mean square) three-dimensional displacement of a He atom in one day (86,400 s) if it remains in a Ne gas atmosphere?
A. 4.02 m
B. 13.7 cm
C. 2.32 cm
D. 162 km
Answer
C. move left ... when Δc = 0
Entropy will drive the K+ ions to occupy the maximum
volume available because that is the most disordered state.
C. move left ... when ΔcK+ = 0
Entropy will still cause the K+ ions to occupy the maximum
volume available because that is a more disordered state than if they were to stay on the right side
of the partition. The Na+ ions are not free to move across the membrane so they stay put.
The final situation looks like:

D. 8.46 h

Answer B is the only one with the correct units cm2/s. It is similar to equation 19.53 p. 689
of the Physical Chemistry book by David W. Ball.

D. twice
Because the concentration difference Δc = c0 − 0 does not change, the gradient Δc/Δx in the Δx = 30-cm-long tank is twice the gradient of the Δx = 60-cm-long tank. Doubling the concentration gradient will double the diffision rate ΔN/Δt.
A. 4.02 m



 A. move left ... after an infinite time
A. move left ... after an infinite time A. move left ... after an infinite time
A. move left ... after an infinite time
 A. one-quarter
A. one-quarter


