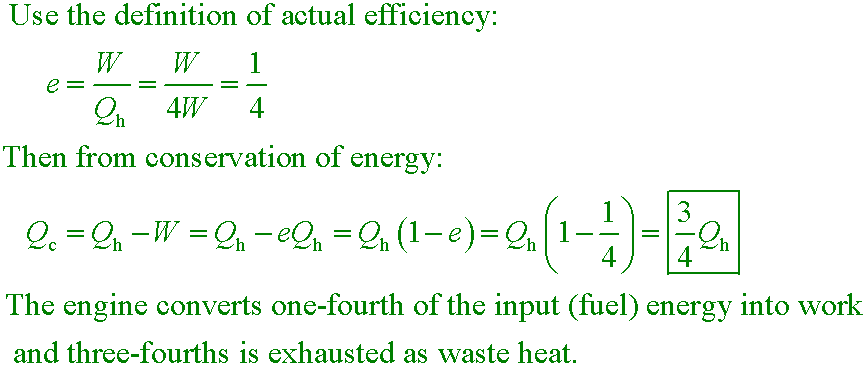Summary
- Heat engines
- Refrigerators
- Carnot cycle
pop4 qq18.1
The energy input to an engine is 4.00 times greater than the work it performs. For this engine,
what fraction of the energy input is expelled to the cold reservoir?
A. 0.250
B. 0.375
C. 0.500
D. 0.750
Answer
Walker4e
Reading quiz An increase in entropy is the same as _________.
A. a decrease in order
B. a decrease in internal energy
C. an increase in order
D. an increase of thermal energy stored in molecular interactions
Answer
kw4
A bunch of water is placed in a freezer and becomes a block of ice. The entropy of the water has
A. increased.
B. decreased.
C. stayed the same.
Answer
kw4
A ringing bell is inserted into a thermally insulated container of water.
The bell and the water initially have the same temperature. Eventually the bell stops
ringing and the water stops moving. What happened to the mechanical energy of the bell?
A. It became potential energy.
B. It became internal energy.
C. It escaped from the system.
D. It lowered the entropy.
Answer
kw4
What happened to the temperature of the water and the bell?
A. It went up.
B. It stayed the same.
C. It went down.
Answer
kw4
What happened to the entropy of the system?
A. It went up.
B. It stayed the same.
C. It went down.
Answer
kw4
Why won't the bell start ringing again when removed from the water?
A. Not enough energy is present.
B. The water isn't warm enough.
C. It would require an entropy increase.
D. It would require an entropy decrease.
Answer
sj6 22.36
Liquid helium boils at 4.20 K with Lv = 20.5 kJ/kg. What is
DS per kg for vaporizing helium?
A. 4.20 kJ/kg·K
B. 4.88 kJ/kg·K
C. 20.5 kJ/kg·K
D. 86.1 kJ/kg·K
Answer
sj6 22.40
What entropy change occurs when 1000 J of energy is transferred by radiation from the Sun to the Earth?
Tsun = 5700 K, TE = 290 K.
A. −0.370 J/K
B. 5.70 J/K
C. 3.45 J/K
D. 3.27 J/K
Answer
D. 0.750
A. a decrease in order
The textbook explains in detail in section 18-9 how entropy
can be thought of as a measure of the amount of disorder in a system.
B. decreased.
Energy has left the water/ice so its entropy has decreased. However, the entropy of the universe has increased as a result of operating
the refrigerator that created the ice. Whenever ice spontaneously forms in nature it is because heat has flowed from hot to cold,
a process that always increases the entropy of the universe.
B. It became internal energy.
The friction (viscosity) in the water converted the mechanical energy into heat.
A. It went up.
An increase in internal energy usually produces an increase in temperature, unless a phase change (melting, boiling, etc.) is
occurring.
A. It went up.
Heat was deposited into the water and the bell, increasing the entropy of both. Energy has thus been converted from mechanical
(ordered) to thermal (disordered) energy.
D. It would require an entropy decrease.
Heat would have to be removed from the water and the bell and spontaneously form into an ordered
energy. In principle this could happen but it never does because it is so unlikely.
B. 4.88 kJ/kg·K

D. 3.27 J/K