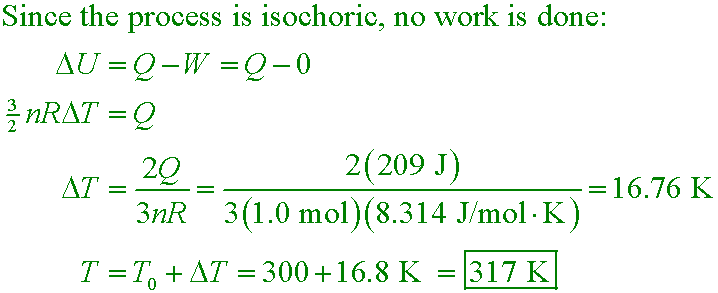Summary
- Laws of thermodynamics
- Thermodynamic processes
sj6 21.15
209 J of energy is transferred by heat in a constant-volume process to 1.00 mol of a monoatomic
ideal gas that is initially at 300 K. What is the final temperature?
A. 509 K
B. 312 K
C. 328 K
D. 317 K
Answer
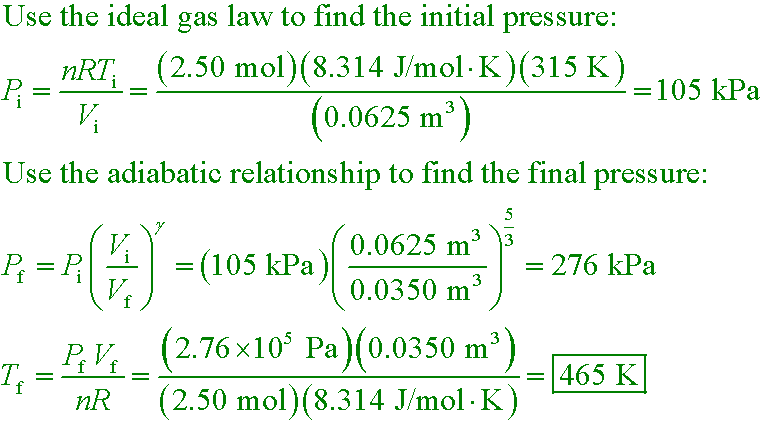
wlk4 Ex.18-5
A 0.0625-m3 container holds 2.50 moles of a monatomic ideal gas at a temperature
of 315 K. The gas is now compressed adiabatically to a volume of 0.0350 m3. What
is the final temperature of the gas?
A. 176 K
B. 315 K
C. 465 K
D. 788 K
Answer
D. 317 K
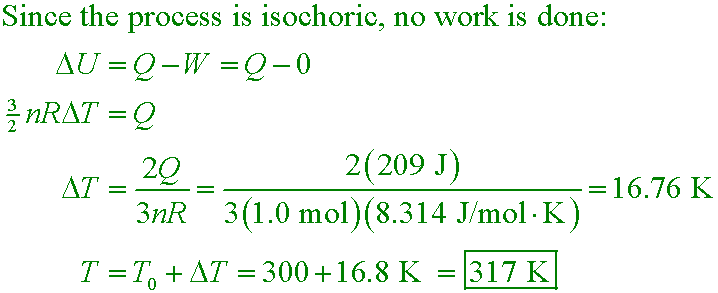
C. 465 K