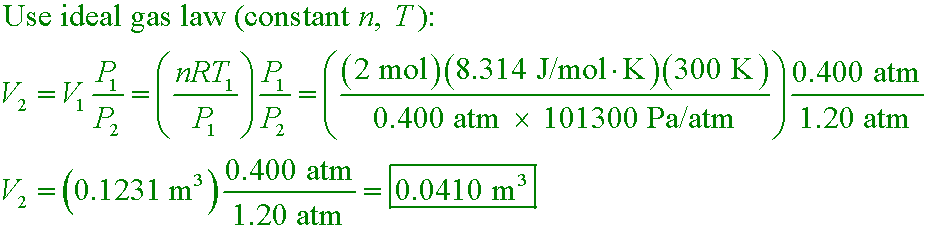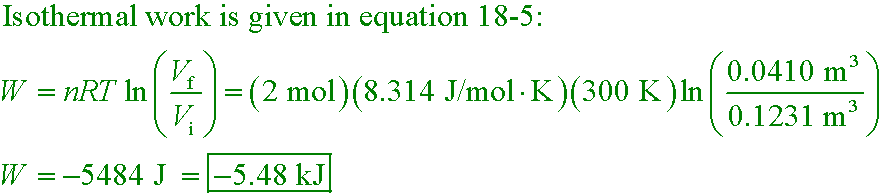Summary
- Kinetic theory of gases
- Elastic properties of solids
- Latent heat
- Class Quiz Timer
- Reading Quiz
- Thermodynamic processes
- W = PΔV
- PV diagram applet
- isothermal (constant temperature)
- isobaric (constant pressure)
- isovolumetric (constant volume)
- adiabatic (no heat flow)
- process simulator applet
Example #1
- 1st Law of Thermodynamics
kw4
A 100-g cube of aluminum (c = 0.215 cal/g·°C) at −20°C is put
into 300 g of water at 0°C. How much ice will form?
{Lf = 79.8 cal/g}
A. zero
B. 1.43 g
C. 5.40 g
D. 25.1 g
Answer
Walker4e
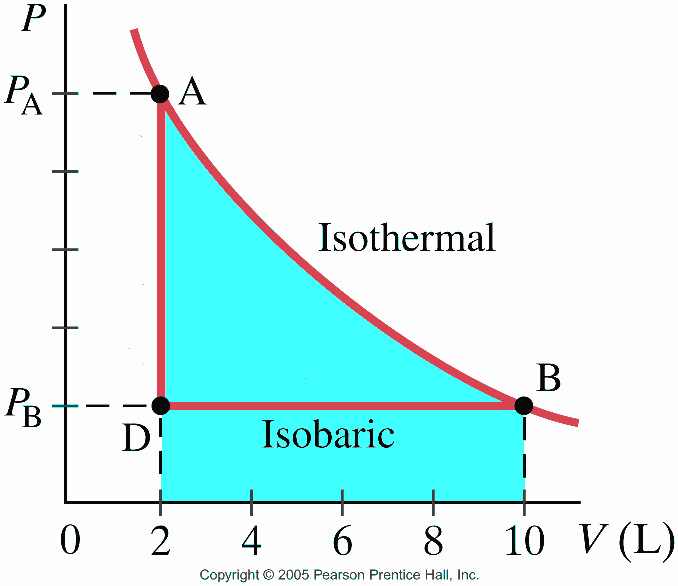
Reading quiz The process depicted in the PV plot shown below is an isothermal one.
What is represented by the shaded area below the curve?

A. The change in thermal energy of the gas during the process.
B. The temperature at which the process takes place.
C. The work done by the gas during the process.
D. The heat capacity of the gas at constant temperature.
Answer
gc6 15.cex15-3
A gas is to be compressed from 2 m³ to 1 m³. Which is harder, compressing it
isothermally or isobarically?
A. Isothermally
B. Isobarically
C. There is no difference
D. It depends upon the initial pressure
Answer
kw4
While a gas is being compressed, 8 J of heat are removed and 20 J of work are done on it.
What is the change in internal energy of the gas?
A. −28 J
B. −12 J
C. +28 J
D. +12 J
Answer
sj6 20.39a
2.00 mol of helium at 300 K and 0.400 atm is compressed isothermally to 1.20 atm. What is the final volume?
A. 0.041 m3
B. 0.123 m3
C. 0.369 m3
D. 2.40 m3
Answer
sj6 20.39b
2.00 mol of helium at 300 K and 0.400 atm is compressed isothermally to 1.20 atm.
What is the work done by the gas?
A. −7.43 kJ
B. −5.48 kJ
C. −3.12 kJ
D. 2.20 kJ
Answer
sj6 20.39c
2.00 mol of helium at 300 K and 0.400 atm is compressed isothermally to 1.20 atm.
What is the energy transferred by heat?
A. −7.43 kJ
B. −5.48 kJ
C. −3.12 kJ
D. 2.20 kJ
Answer
C. 5.40 g

C. The work done by the gas during the process.
The units of force multiplied by distance can be rearranged into
pressure multiplied by a volume change, so a pressure multiplied by a volume change (the area under
a PV plot) represents the work done by the gas. We'll learn later that for an ideal gas to
maintain its temperature while expanding, an amount of heat must be added to the gas that is equal to
the work done by the gas, that is, equal to the same area depicted in the figure.

A. Isothermally
The pressure rises for isothermal compression, so more work is required to compress the gas. For
an isobaric compression the temperature must decrease, and the pressure stays low during the entire process, so less work is required.
D. +12 J
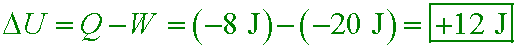
A. 0.041 m3
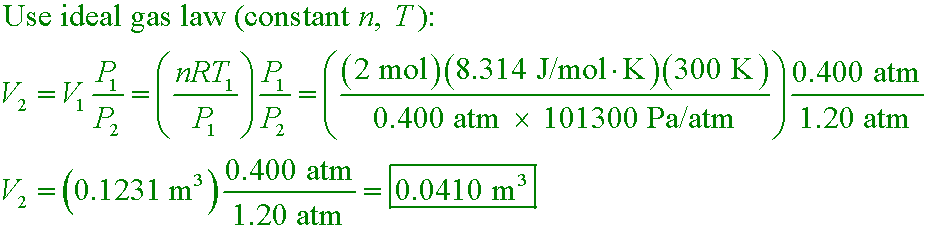
B. −5.48 kJ
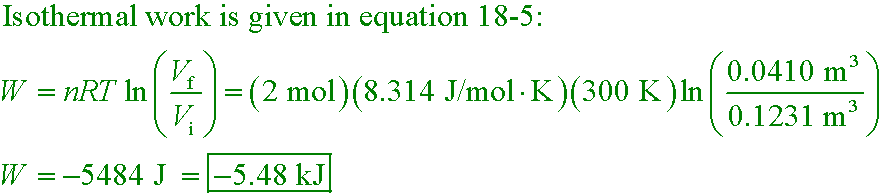
B. −5.48 kJ
The heat released must equal the work done on the gas in order to
keep the temperature of an ideal gas the same.