Summary
- Kinetic theory of gases
- Elastic properties of solids
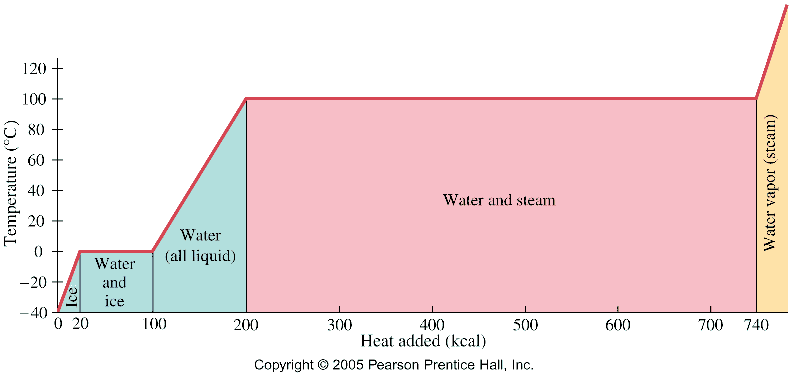
- Latent heat
- Reading Quiz
- Latent heat
Example #1
Chapter 18
- Laws of Thermodynamics summary
- 0th Law: thermal equilibrium
- 1st Law: conserve energy
- 2nd Law: increase entropy
- 3rd Law: T > 0 always
Walker4e
Reading quiz One gram of steam at 100°C that comes in contact with your skin will
cause a more serious burn than one gram of water at 100°C because
- the steam first emits the latent heat of vaporization as it condenses to water.
- the steam requires the latent heat of fusion to be delivered by your skin.
- the steam chemically reacts with your skin cells but the water does not.
- the steam has a higher specific heat than water and therefore delivers more energy.
Answer
klm
How many −10°C, 25-g ice cubes should you add to cool a 2-L bottle of warm (25°C) soda
down to 5°C? Assume csoda = cwater
A. 4
B. 7
C. 10
D. 18
Answer
A. the steam first emits the latent heat of vaporization as it condenses to water.
A gram of steam will emit 539 cal when it condenses to water at
100°C, and another 63 cal as it cools down to 37°C. A gram of water at 100°C will only
deliver the 63 cal as it cools down. So the steam delivers almost ten times more energy than does
the water, causing a much more serious burn.
D. 18



