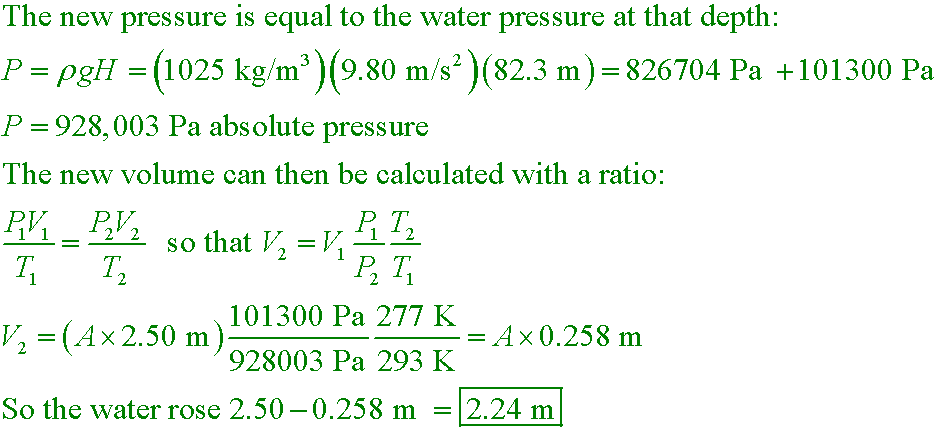Lecture 36
Summary
- Thermal expansion
- Specific heat
- Conduction, convection, radiation
Walker4 16.31
Two objects made of the same material have different temperatures. Object A has mass m
and object B has mass 2m. If the two objects are brought into thermal contact,
- object B gives up more heat and changes its temperature more than object A.
- the heat released by one object is absorbed by the other, and the temperature change of each
is the same because the specific heats are the same.
- the heat released by one object is absorbed by the other, and the temperature change of object A is
larger than the temperature change of B.
- object A changes its temperature less than object B because the heat exchanged per mass must be
the same for each.
Answer
Walker4e
Reading quiz Feeling a bit cool, you turn up the thermostat in your living room. A short time
later the air is warmer. If the room is well sealed, the pressure of the air is _____ it was before
you turned up the heat.
A. greater than
B. less than
C. the same as
Answer
klm
The absolute temperature of an ideal gas is _____ if the volume of the sealed container is halved at constant pressure?
A. cut to ¼
B. cut to ½
C. the same
D. doubled
Answer
sj6 19.44
A 2.50-m-high diving bell is lowered into 1025 kg/m3 sea water at 20°C. At 82.3 m of depth and 4.0°C, how high
does sea water rise into the bell?
A. 2.50 m
B. 2.24 m
C. 1.87 m
D. 0.26 m
Answer
gc6 q13.16
When an ideal gas at 300 K expands against a piston its volume doubles and the pressure drops to 38% of its
initial value. What is the final temperature of the gas?
A. 300 K
B. 394 K
C. 1579 K
D. 228 K
Answer
gc6 q11.20
N2 molecules are lighter than O2 molecules. Do you expect more N2 or
more O2 at high altitudes in Earth's atmosphere?
A. more N2
B. more O2
C. the same ratio as at low altitude
Answer
sj6 21.4
A 5.00-L vessel contains 2 mol of oxygen gas at P = 8.00 atm. Find the average translational kinetic energy
of the O2 molecules.
A. 1.74×10−19 J
B. 5.03×10−21 J
C. 6.82×10−25 J
D. 3.19×10−27 J
Answer
sj6 21.42
A gas is at 0°C. What DT is required to double vrms?
A. 4°C
B. 199°C
C. 273°C
D. 819°C
Answer
C. the heat released by one object is absorbed by the other, and the temperature change of object A is
larger than the temperature change of B.
Suppose object A is hotter. It loses heat of magnitude Q and
object B gains heat of magnitude Q. With the same magnitude of heat involved, and the fact that
the specific heats are the same, you can see from Q = mcΔT that the smaller object A
will change its temperature by a larger amount than the larger object B.
A. greater than
Higher temperature means the molecules will have a greater kinetic
energy and will collide with the walls of the room more often and with greater force. We therefore
expect the pressure to increase. If the pressure were to be kept constant, air would leave the room so
that fewer molecules will collide with more force with the walls. You can also think of this in terms
of the thermal expansion of air.
B. cut to ½

B. 2.24 m
D. 228 K

A. more N2
Imagine each molecule is launched upwards with the same kinetic energy
(because they are at the same temperature). They will each
gain the same amount of gravitational potential energy mgy, but N2 will achieve a
higher altitude y because it has a smaller mass m.
B. 5.03×10−21 J

D. 819°C