Summary
- Thomson model of the atom
- Rutherford model
- Blackbody radiation
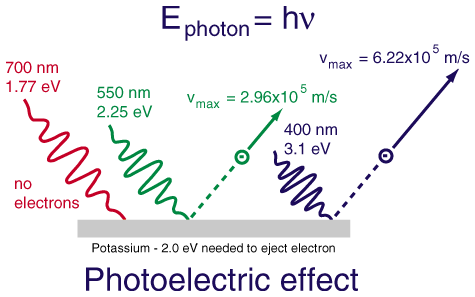
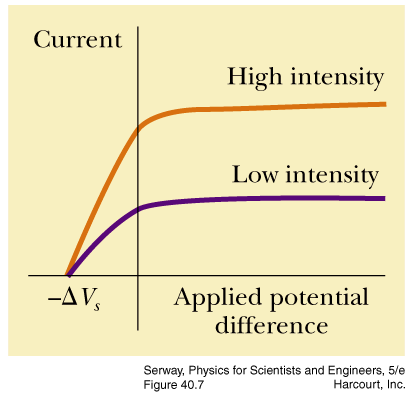
- The photoelectric effect applet
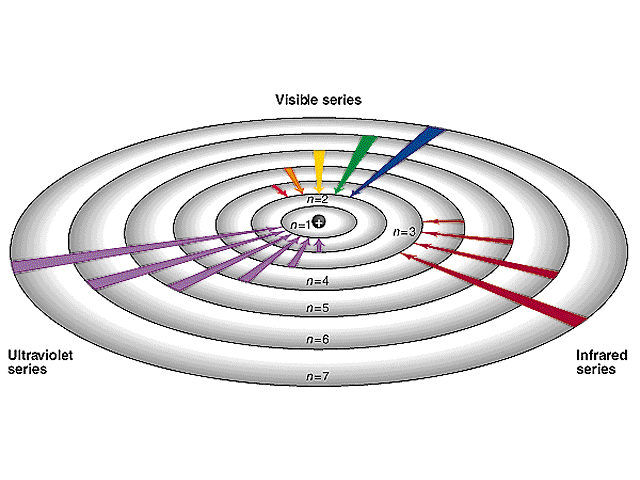
- Bohr model of the atom
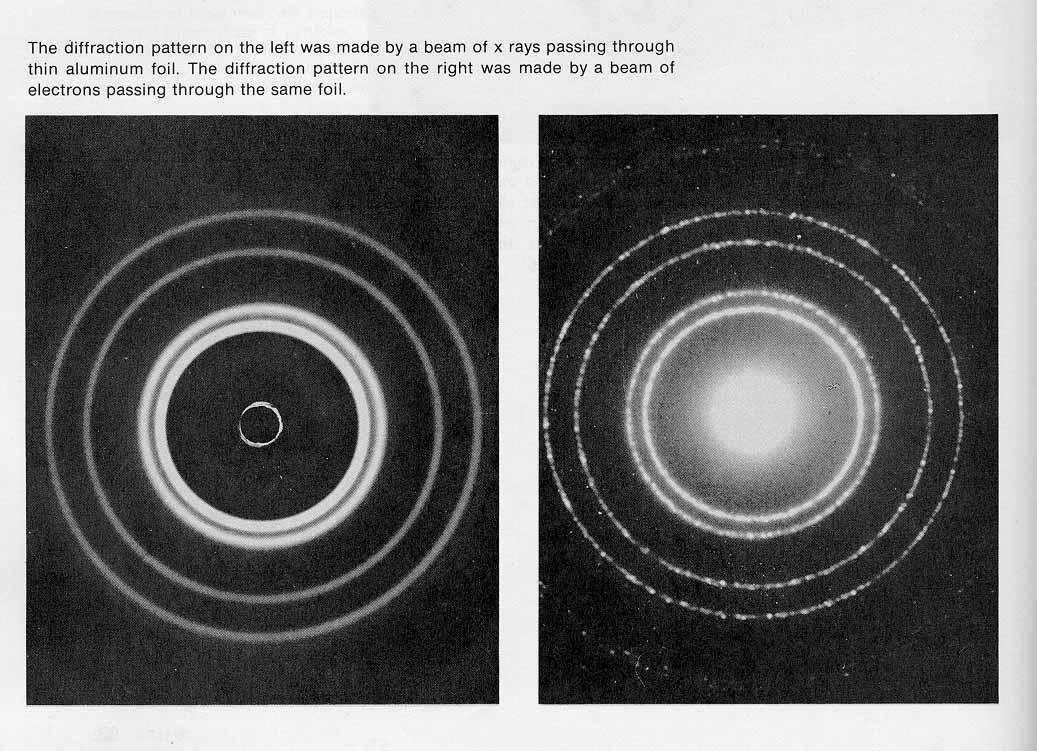
- de Broglie waves
- particles can behave like waves
- de Broglie wavelength
Example #5
- electron diffraction experiment
- your wavelength

kw5
Light striking a clean metal produces photoelectrons. If the light is made twice as bright, what happens to the average
kinetic energy of the photoelectrons?
A. The energy doubles.
B. The energy stays the same.
C. The energy is cut in half.
D. It's impossible to say.
Answer
kw5
Blue light shines on a metal, producing photoelectrons. When a red light is used the maximum KE of the photoelectrons
A. increases.
B. decreases.
C. stays the same.
D. Depends on the light intensity.
Answer
Which of the following is NOT a feature of the Bohr model of the atom?
A. Electrons in planetary-like orbits.
B. Positively charged material surrounding the electrons.
C. Quantized energy levels.
D. Accelerating electrons that do not radiate.
Answer
If the electrons in hydrogen atoms are excited to the fourth Bohr orbit, how many different frequencies of light may be emitted?
A. 1
B. 3
C. 4
D. 6
Answer
If an electron and a proton have the same speed, which would have the longer wavelength?
A. The electron.
B. The proton.
C. They would have the same wavelength.
Answer
B. The energy stays the same.
The average kinetic energy of the emitted photoelectrons depends only upon the frequency of the light. Making the light brighter
produces more photoelectrons, but they have the same energy distribution as before.
B. decreases.
Red light is composed of photons that have a lower frequency and thus less energy than blue photons. The emitted photoelectrons
will therefore have less energy when red light is used.
B. Positively charged material surrounding the electrons.
D. 6
A. The electron.